 The Top Ten Mineral Localities on Earth
The Top Ten Mineral Localities on Earth
Philip M. Persson
3139 Larimer St., Denver, CO, 80205
perssonrareminerals.com
Graduate Student, Dept. of Geology & Geological Engineering
Colorado School of Mines
I have often had collectors ask me what localities I consider to be ‘important’ or ‘world-class’ as far as diversity, quality and quantity of minerals, and I’ve also spent much time pondering this question myself. In the end, the answer is highly subjective, depending on the person’s interests and experience in mineralogy and what drives them as collectors. There are ‘mineralogical rainforests’ such as Mont Saint Hilaire, Canada or Russia’s Kola Peninsula which, while not well-known for large aesthetic crystallized specimens, host an incredible diversity of mineral species- a seemingly infinite combination of a finite set of elements which attests to the unique geologic conditions under which they form. Then there are locales that have produced iconic and beautiful examples of one or perhaps a few minerals, but are otherwise fairly ‘simple.’ Colorado’s Sweet Home Mine or the Elmwood Mine in Tennessee likely fall into this category. In my opinion, the best localities are those that successfully bridge the gap between these extremes; those that have produced beautiful, highly collectible crystals but also have a deep appeal to the academic mineralogist or serious systematic collector. The following is a brief, somewhat arbitrary list of what I consider to be 10 of the top such locales, and I hope you enjoy my musings on each mineralogical treasure chest. –Phil Persson, Denver, Colorado December 2015

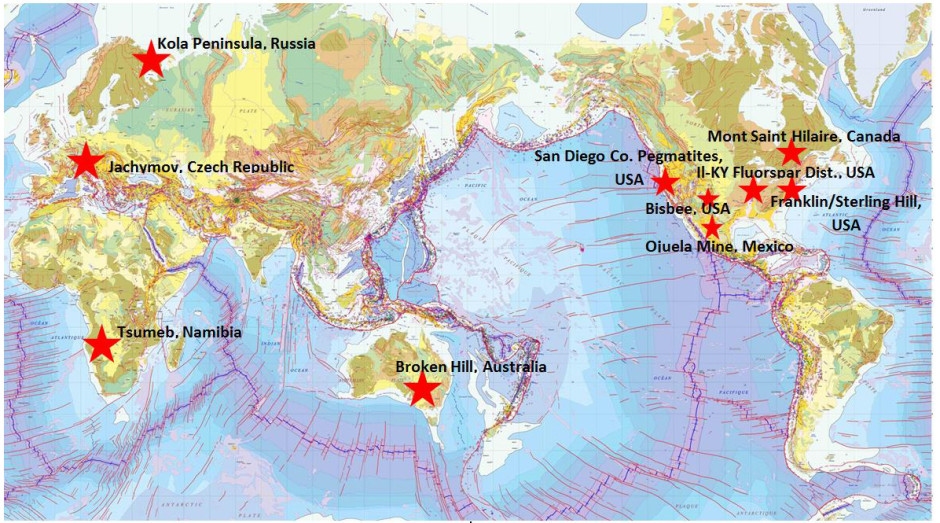
1.) Franklin & Sterling Hill, Sussex County, New Jersey, USA

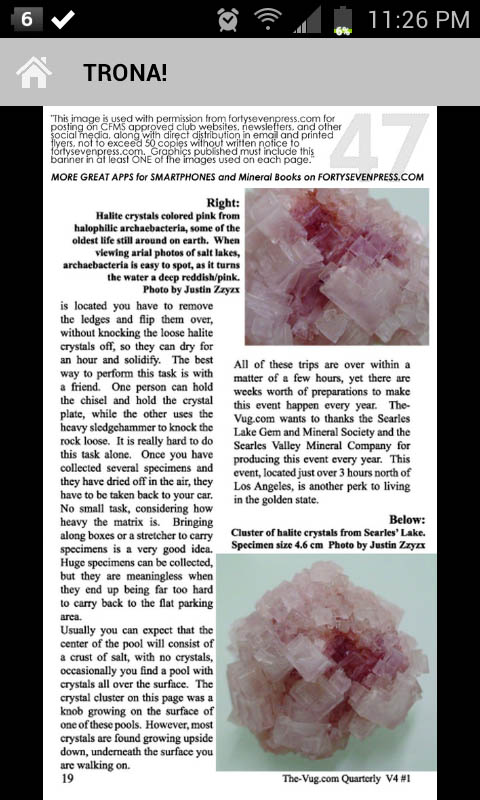
Willemite & Franklinite crystals, Franklin Mine, 15 cm across (photo © kristalle.com, Tucson Show 2008)
Franklin. The name instantly kindles an affectionate smile or nod from seasoned rare species or fluorescent mineral collectors, and perhaps a begrudging acknowledgment from collectors of aesthetic minerals like gem crystals. No matter your interests, however, the unique appeal of Franklin (and it’s slightly smaller sister deposit, Sterling Hill) cannot be denied. These two mines, both now closed, are situated in the rather bucolic Northwest corner of the much-maligned state of New Jersey, approximately 45 airline miles from New York City. The unique and varied mineralogy of Franklin & Sterling Hill (over 350 mineral species now known, a number exceeded only by Mont Saint Hilaire and a German mine whose mineral endowment can be equally attributed to the forces of nature and the persistence of German micromounters) can be attributed to the unusual forces that led to their creation.

Passaic Open Pit with Sterling Hill Orebody under shortwave UV light at night (photo © Sterling Hill Mining Museum)
Originally thought to be seafloor volcanogenic massive sulfide deposits (previously termed ‘exhalites’), which formed near ‘black smoker’ type hydrothermal vents in a rifting environment in the late Proterozoic period, the Franklin & Sterling Hill orebodies were later subjected to fairly high-grade regional metamorphism (upper amphibolite facies) which turned the surrounding carbonate rocks into crystalline marbles, and transformed the fairly benign sulfide mineralogy of the proto-deposits into the exotic mixture it is today; defined principally by the Zn-Fe-Mn oxide Franklinite, the Zn-silicate Willemite, and the Zn-oxide Zincite. None of these minerals can be considered ‘common’ on a global scale, and two are essentially unknown outside the district. Metamorphism also introduced a suite of igneous intrusives of varying composition, from basaltic ‘camptonite’ dikes to felsic pegmatites with their own metasomatic reactions and accompanying fluids, which altered and further complicated the already diverse mineral assemblages present.
I will not delve too deeply into the history of the Franklin-Sterling Hill District, which could (and does) occupy an extensive treatise unto itself- suffice it to say that the orebodies, which originally outcropped in a fairly spectacular fashion, have been known since at least the late 17th century and probably earlier, but metallurgical issues arising from the complexity of the ores as well as disorganization of various mining entities prevented large-scale mining until the later part of the 19th century. From ~1880 to WWII was the ‘heyday’ of Franklin & Sterling Hill, with extensive scientific investigation of both deposits by many of the leading mineralogists of the day, such as Charles Palache & Clifford Frondel. Numerous new minerals to science were described, and the genesis of the deposits was fiercely debated. The combined output of the two mines, in total some ~40,000,000 tons of ore averaging >20% Zn (grades generally unheard of today) and substantial Fe & Mn propelled the owner, the New Jersey Zinc Company, into the upper leagues of the mining industry and allowed them to expand all over the U.S and the world (Dunn 1995). When the Sterling Mine finally brought its last ore bucket to the surface in early 1986, the district had a several hundred year history of mineral collecting and mineralogical science, as well as a robust ‘local scene’ with fierce competition for choice specimens and a club which fostered the community through mineral shows and other events.
It would take another monograph (this exists as well, authored by former-Smithsonian mineral guru Dr. Pete Dunn, and is a must-have for the serious Franklinophile) to describe the minerals of Franklin & Sterling Hill in detail, so I will just say a few words about some of the more notable species. At the top of the list of course are the ore minerals: franklinite, willemite, and zincite. With the exception of willemite (and this is debated amongst some), Franklin & Sterling Hill have produced by far the world’s premier crystallized examples of these species; in atypically-attractive euhedral crystals, up to 20 cm. on edge for Franklinite, and similarly large (or larger) for willemite. Willemite is a ‘chameleon’ at Franklin-Sterling Hill and occurs in nearly all imaginable colors and textures. Early 20th-century New Jersey Zinc Company chemist Lawson Bauer had a box of over 50 specimens (now in Harvard University’s collection) he would often have visitors try and identify. The trick is they were all willemite! Willemite even occurs rarely as flawless, gemmy prisms in attractive shades of green and yellow to blue to 4 or 5 cm. in size, which any real Franklin collector would murder their grandmother for. Zincite also occurs as sharp blood-red pyramids up to 5 cm, though 99% of it is massive.
Next in importance and distribution in collections are probably the Mn-bearing and so-called ‘skarn minerals’, Rhodonite being the most important. Broken Hill, Australia or Brazil have perhaps produced gemmier and more lustrous Rhodonites, but as far as sheer abundance, diversity and mineral associations, nowhere can beat Franklin. Attractive pink to red rectangular prisms to 20+ cm. embedded in white calcite matrix associated with willemite and franklinite crystals comprise the Franklin ‘uber-classic.’ The best ones have been painstakingly excavated from their enclosing calcite using small dental picks and hammers. Bustamite also occurs in excellent crystallized examples, as well as a host of much rarer Mn-bearing species such as Johannsenite, Hodgkinsonite, and Leucophoenicite (all having their type-locale at the Franklin mine). Species such as these illustrate that truly ‘world class’ localities exhibit the geochemical attribute of having a mineral representing essentially every thermodynamically-stable combination of a ‘signature’ set of elements. In the case of Franklin & Sterling Hill, these elements include Zn, Mn, Fe, Si, As, O, Ca, B, Pb, Ba, and a handful of others. None of these is particularly rare in the earth’s crust, but when combined in enough ways, new minerals to science which are globally-scarce are bound to result.
No discussion of Franklin would be complete without mentioning the fluorescent minerals. Mineral fluorescence, a spectacular property some minerals exhibit when certain outer shell (or ‘valence’) electrons are energized and emit vibrantly-colored visible light colors when excited by ultraviolet light sources, perhaps reaches its global zenith at Franklin. Over 80 minerals found at Franklin & Sterling Hill fluoresce under UV light, and many in bright and attractive combinations of color known the mineral world over. The cause for such a diversity of fluorescent minerals has been much debated, but probably involves the metamorphic and geochemically-complex nature of the deposits, as well as the abundance of certain elements such as Mn & Pb thought to act especially well as ‘activators’, or elements receptive to U.V light-induced excitation of key electrons. One of the major ore minerals at Franklin & Sterling Hill, willemite, fluoresces bright green under shortwave U.V light, and the major gangue mineral for both deposits, calcite, fluoresces bright red due to trace manganese, and together these minerals form a vivid fluorescent combination known locally as ‘red and green.’ This material has made its way into mineral collections around the globe, as have examples of minerals like hardystonite, esperite, margarosanite, clinohedrite, hydrozincite, sphalerite, roeblingite, and more. Some of these minerals, like roeblingite, margarosanite, and manganaxinite, are rare and restricted in their occurrence even at Franklin, and are highly-coveted by collectors even in modest examples. Others such as willemite, calcite and hydrozincite can still be easily collected today and are sure to delight any first-time visitor to the area.
Finally, the huge diversity of ‘rare’ species must be acknowledged. I put rare in quotation marks because it is a relative term at these enigmatic deposits, but some minerals are ‘truly rare’ and are unknown outside Franklin/Sterling Hill, or have global amounts totally a few grams of micro-crystals. It would be futile to describe these in detail here, but suffice it to say that they have names like Gerstmannite, Walkilldellite, Hauckite, Ogdensburgite, Kraisslite, Cahnite, Charlesite, and Samfowlerite which pay homage to their shared type locality and the scientists and collectors whose passion for the district led to their discovery. While the mines of Sterling Hill & Franklin have been closed since 1986 and 1954 respectively, their legacy is proudly carried on by two superb museums and a small army of local collectors and aficionados who almost without thinking refer to all rocks not from the area as ‘foreign.’
2.) Mont Saint Hilaire, Quebec, Canada

Serandites to 15 cm in the Royal Ontario Museum (photo © Dave K. Joyce)
First Franklin, now Mont Saint Hilaire!? Surely, the collectors of aesthetic and dare I say, “normal” minerals are now really rolling their eyes and assuming I must have some hidden ugly mineral agenda. But wait! Have you not seen the lustrous, bright orange 20 cm. Serandite crystals studded with lustrous white analcime golf balls? Or the lemon-yellow tablets of bright Catapleiite with swords of lustrous red maganeptunite shooting out of them? Or the brilliant-blue cubes of carletonite? Or perhaps the gemmy red crystals of Rhodochrosite on a bed of shiny natrolite prisms? Not everything at the ‘super classic’ pair of quarries nestled in an enigmatic hillside in the Quebec countryside requires a microscope to see. But, for those with an eye for the rare and unusual, Mont Saint Hilaire truly opens up another universe, with 400+ known mineral species and more awaiting proper documentation.

aerial photo of Poudrette Quarry ( photo © McGill University)
Mont Saint Hilaire (MSH) is a globally-significant example of a multi-phase alkalic intrusion, an unusual type of igneous rock typically associated with either extensional tectonism (rifting) or hot spot activity, both of which have the capability to bring deep-seated, ‘primitive’ mantle magmas enriched in rare elements to the surface. In the case of Mont Saint Hilaire, the unusual alkali intrusive complex formed approximately 120 years ago when the New England hot spot, a relatively fixed, long lived mantle-plume like conduit for deep magmas ascending to the surface, erupted a series of intrusive and extrusive igneous rocks as the North American plate slid westward over it (Currie et al. 1986). In the vicinity of Mont Saint Hilaire, these complexes are known as the Monteregian Hills, and include other famous mineral localities such as the Francon Quarry on Montreal Island and the Oka Carbonatite complex just to the north. At Mont Saint Hilaire, the igneous intrusion consisted of a highly diverse suite of rocks ranging from gabbro at the mafic end to nepheline syenite at the felsic, critically silica-undersaturated end. The surrounding ‘country rocks’, mainly Paleozoic carbonate rocks, were also thermally ‘cooked’ by the intruding magma, and produced a contact-metamorphic rock type known as hornfels (Currie et al. 1986). The most interesting rock type produced during this intrusion are the pegmatites, which are often highly alkaline (e.g; enriched in elements such as Na & K) and contain a huge diversity of minerals (one pegmatite contained over 100 minerals!) due to the ability of such late-stage melts and associated hydrothermal fluids to transport large amounts of typically incompatible elements, such as Ti, Zr, Nb, & REE.
The history of Mont Saint Hilaire as a mineral locality is relatively recent and started in earnest after WWII. The mountain, which is quite prominent and sticks up ~400 meters above the surrounding flat, agricultural countryside, was long before noted for its unusual igneous rocks, but generally poor exposure precluded any notice of its unusual mineralogy. Industrial-scale quarrying for road metal for the nearby city of Montreal changed this situation dramatically, when a large body of layered and zoned nepheline syenite and associated agpaitic rocks loaded with rare minerals were exposed in the 1950’s and 60’s. Quarrying, as well as scientific study of the mineralogy and geology of the mountain intensified in the 1970’s, and probably reached its zenith in the early 1980’s, at least as far as recovery of fine specimens went. Blasting and rock moving happened at large scales on a daily basis, and the owners of the main quarry, the Poudrette family, was accommodating to collectors as well as scientists. Stories of ‘walk in’ (or crawl-in) pockets abounded, and one pocket filled with amazing crystals of serandite, luecophanite, neptunite, analcime and more was said to be almost 10 meters deep and 2 meters wide, easily swallowing several collectors at a time! Collecting during these times was easy and fantastic; all one needed to do was scoop crystals into a waiting flat or bucket out of recently blasted pockets or pegmatites, and sort through it later for rare (and sometimes new!) species. One friend of mine recounted a story once of how after collecting a huge pocket filled with world-class Serandite crystals in the 1980’s, the Royal Canadian Mounted Police later paid him a home visit at his residence south of the border to inform him that his find constituted national Canadian heritage and was to be returned at least in part to Canada!
Sadly, all good things must come to an end, and in the case of Mont Saint Hilaire it had less to do with geology and mineralogy and more with the whims of quarry management and demand for aggregate rock. The quarry slowly moved away from the more productive, pegmatite-rich zones of the intrusive complex and into the hornfels, and finally into the ‘barren’ Paleozoic sedimentary rocks of the St. Lawrence lowlands. This move, coupled with restrictions on collector access leading to long periods of essentially no access to newly-quarried exposures, meant that many wrote off MSH as being a ‘dead’ locality. The mountain is far from exhausted however as far as its mineralogical treasures are concerned, and perhaps a new, more scientifically-receptive ownership or a change in demand for road material will awaken a new era for this truly world-class mineral locality.
Mineralogically it is impossible, as with Franklin, to briefly summarize the abundance and diversity of minerals. Suffice it to say that as with other ‘hyper-alkaline’ igneous complexes like the Lovozero Massif in Russia and Dara-I-Pioz in Tajikistan, the ‘characteristic’ elements associated with the intrusions, such as Na, Zr, Ti, Fe, F, REE, Nb, & Y occur in seemingly endless combination, leading to a huge number of different elements essentially assembled from a restricted set of elements under restricted by sufficiently diverse geologic conditions. Many of these occur of course as micro-crystals whose best euhedral development does not exceed a few millimeters, and often much less. But, equally impressive are number of minerals which occur as colorful, euhedral crystals AND are extremely rare or sometimes even unknown outside MSH.
The ‘poster child’ (and indeed, it has been on many posters) is Serandite, the Mn-rich cousin of pectolite, which was first discovered on the isle of Rouma in Equatorial Guinea, itself an alkalic intrusion, but reaches its peak at MSH, where it forms lustrous, sometimes even gemmy salmon-pink to vibrant orange crystals to 20+ cm. Often associated with these are crystals of analcime, itself not a rare mineral but especially well-crystallized at MSH, where lustrous, sharp baseball-sized crystals are not unusual. Catapleiite, another unusual mineral (and part of the well-represented zirconosilicate family of minerals from MSH), forms superb, sometimes gemmy crystals to 10 cm, sometimes associated with other rare species. Carletonite, first discovered at MSH, occurs as beautiful, sky-blue crystals to several cm., often in attractive groups. Aegirine, Natrolite, Manganeptunite, Leucophane, Siderite, Rhodochrosite, Genthelvite, Donnayite-(Y), and Eudialyte are all notable species for the locality, some reaching their best here. While access at the moment is restricted to Mont Saint Hilaire, the large pegmatite-rich zone of the intrusion is far from exhausted, and new quarrying to supply the growing city of Montreal will surely expose new world-class minerals.
3.) Broken Hill, New South Wales, Australia

Rhodonite: 17 Level, Zinc Corporation Mine, Broken Hill- 4.7 cm. high, (photo by Carl Bento, © Australian National Museum)
Broken Hill is a world-class deposit in every sense of the word. Economically, it is one of the largest single mineral deposits on earth and helped start a company that it now Australia’s largest (BHP Resources). Scientifically, it is a global enigma which has puzzled economic geologists and mineralogists for generations. Culturally, it paved the way for the success of ‘frontier mining towns’ of the Australian interior which became essential to creating what is now a nation with one of the highest standards of living in the world. While not nearly operating at its former pace, the district is still the site of active mining for lead, zinc, copper, silver and gold under mid-sized mining company Perilya Resources, and exploration for new major deposits in the area is still actively underway.
Geologically, Broken Hill is classed as a metamorphosed volcanogenic massive sulfide (VMS) type deposit. It began as a large, essentially flat-lying body of Zn, Pb, Cu, & Ag sulfides with other metal enrichment on the seafloor at the site of extensive and long-lived hydrothermal venting during rifting on the ocean floor in the Paleoproterzoic period, almost 2 billion years ago. Like much of Australian, the larger geologic history of the region dates far back into Archean time 2+ billion years ago, and is one reason the continent is so incredibly mineral-endowed. After ‘primary’ sulfides were deposited as a combination of ‘exhalite’ seafloor venting and settling of metalliforous hydrothermal fluids and sub-seafloor replacement of receptive host rocks, an additional thick sequence of interbedded volcanic, clastic, and sedimentary rocks were deposited, which eventually became known as the Willyama Supergroup, and important formation in the western part of New South Wales and northern Victoria (Marjoribanks et al. 1980). Around 1.6 billion years ago, the entire rock package was subjected to regional high-grade metamorphism at up to granulite facies, at 750-800 degrees C and 5-6 kilobars pressure Victoria (Marjoribanks et al. 1980). The relatively simple sulfide orebodies as well as their host rock were ‘cooked’ and recrystallized into the diverse mineralogy seen today, and perhaps more importantly, as the metamorphic process waxed and waned and new hydrothermal and magmatic fluids were introduced, the whole system was able to ‘stew in its own juices’ (to quote Brian England) and develop an even more unusual geochemistry. The resulting deposit, once a flat-lying layer on the seafloor, was then folded and refolded into the complex geometry seen today, with the result being a series of stretched, steeply-dipping ‘lenses’ which culminated in a large surface outcrop of leached and altered ore, the original ‘Broken Hill.’

#2 Shaft, North Mine, Broken Hill (photo © Rod Wilkinson/flickr.com)
The human history of Broken Hill is likewise fascinating. While sporadically settled and travelled through by aboriginal peoples for thousands of years, Anglo settlers first visited the area in the 1840-50’s, though the outcrops of leached ore hinting at the bonanza hidden below did little to inspire early pioneers and prospectors. Finally, in 1883, itinerant prospector Charles Rasp staked several claims on the outcrops known as the ‘Broken Hill’ and later with his ‘syndicate of seven’ other prospectors, founded the Broken Hill Proprietary Company, or BHP (Worner 1982). By 1885, mining along the ‘line of lode’, or the main structural trend connecting mineralized lenses was well underway, and Rasp and his partners had realized a many, many fold profit on their investment (Worner 1982). Soon, Broken Hill grew to a respectable city of over 25,000, with all the accouterments associated with more genteel cities of the coast like Melbourne and Sidney. Mining probably peaked around WWII and has been in slow decline since as the main orebodies, mined in places to almost 3500 meters depth, are slowly exhausted. New exploration, however, could be promising since structural complexities and post-ore faulting mean that a large portion of the original sulfide seafloor VMS deposit could have been later isolated from the main ‘line of load’ and lie undetected at depth. Deposits such as Broken Hill often display fabulous ore grades, with 30+ % combined Pb and Zn not unusual, and significant Cu, Ag, and Au adding value as well. So, while Broken Hill has evolved into a quasi-tourist destination and a modern small outback city, it may once again boom day and night with the turning of sheave wheels and lifting of ore buckets to the surface.
Mineralogically, there are over 300 minerals known from Broken Hill, with over 20 of these having first been described there. To collectors, perhaps the best-known are the ruby-red Rhodonite and Spessartine garnet crystals, often embedded in massive galena, which were found at one time in great abundance from the North and South underground mines and other operations. In addition to being beautiful and aesthetic, they speak of the unusual genesis of Broken Hill, in which the original fine-grained banded Pb, Zn, & Mn sulfides were recrystallized under high-grade metamorphic conditions into coarse-grained Mn-silicates and blocky galena. Classic Broken Hill minerals like bannisterite, johansennite, and bustamite formed in a similar manner, reflecting the manganese-rich nature of the original seafloor sediments. Cerussite is another Broken Hill specialty, occurring in superb, large twinned ‘snowflake’ group of reticulated crystals perhaps only exceeded in quality by those from Tsumeb, Namibia. The Sidney museum has numerous cerussite groups to 40+ cm on display, which look almost like miniature cities or MC Escher abstractions, with hundreds of beautiful interconnected crystals at set angles. Anglesite, another secondary lead mineral, also occurs as excellent crystals to over 10 cm, sometimes replacing cerussite.
Excellent examples of malachite and azurite, amongst Australia’s finest, reflect the copper-rich nature of some of the Broken Hill ores, and were found in quantity in the early days when the supergene-enriched near surface ores were mined. Likewise, pyromorphite also forms in the supergene or oxide ores, and occurs as beautiful, sometimes large groups of intergrown prismatic crystals to several cm. in shades of green and brown. Chlorargyrite, an important secondary silver mineral found in the supergene zones, is enriched in the element bromine at Broken Hill, leading to the term ’embolite’ for this chemically-unusual secondary silver mineral. Smithsonite, the zinc carbonate mineral, occurs in a plethora of colors and varieties, as does calcite, which is often colored pink by manganese. On the rarer end of the spectrum, Raspite, Marshite and Miersite were all first discovered at Broken Hill, and all tell of the unusual conditions of secondary ore enrichment at the deposit, containing elements such as iodine, chlorine and tungsten which are somewhat unusual in ore minerals. Hedenburgite, the usually-ugly rock-forming mineral, occurs in wonderful green, lustrous crystals, as does gahnite, the unusual Zn-rich spinel. Orthoclase, colored blue by traces of lead (‘amazonite’) is another speciality, and apophyllite & inesite sometimes occur in beautiful combinations as well. Stolzite, related closely to Raspite, is another classic, occurring as attractive wulfenite-like crystals on black psilomane matrix.
Many more species occur as micro-crystals, and new minerals to the deposit (and science) continue to be discovered to this day. The Kintore open cut, a relatively small open pit operation on one of the few remaining blocks of oxide ore in the 1970’s & 80’s, was the source of many rare species, thanks to systematic efforts of both collectors and company geologists (Worner 1982). Nowadays, good Broken Hill mineral specimens are hard to acquire, both in Australian and abroad. While many if not most miners collected ‘rocks’, many have long-since sold or given away their minerals, and while mining continues, the supply of good minerals appears to be sporadic. With some luck and persistence, good examples of the ‘classic’ minerals like rhodonite and spessartine are still available, but truly top-notch examples will drain your bank account, and examples such as the nearly 6 cm. group of gemmy parallel-growth rhodonites in the Albert Chapman collection, which most collectors would murder their grandmother for, will probably never be found again. But, the sun has not set on Broken Hill yet, and this classic and unique locality may have a new lease on life in the coming years.
4.) Tsumeb Mine, Otjikoto Region, Namibia

Overview of Tsumeb area with famous De Wet Shaft in middle left, May 2014 (Photo © Jean Baptiste, freewheely.com)
The Tsumeb Mine is one of, if not the world’s premier mineral locality. Though its 285+ mineral species already put it in the same league as Mont Saint Hilaire or Franklin in terms of mineralogical diversity, Tsumeb’s true accomplishment is that is has produced thousands of aesthetic, well-crystallized mineral specimens, including arguably the world’s best examples of many perennial collector favorites such as azurite, dioptase, mimetite, cerussite, and smithsonite. This unique juxtaposition of rare species known nowhere else in the world (Tsumeb is the type locality for 71 species) and large, euhedral, colorful crystals is not a coincidence- it is due to an unusual combination of complex geochemistry and development of a massive oxidation zone where supergene enrichment created a paradise for collectors and mineralogists.

Display of minerals (including many fine dioptase specimens) for sale in Tsumeb miner’s Robbie Groebler’s home in 1974, most of which could be had for under $10 (photo © Rock Currier/Mindat.org)
The history of the Tsumeb Mine and surrounding area could be the subject of a full-length treatise on its own, so just a brief contextual sketch will be provided here. Tsumeb is located in the Otjikoto region of northern Namibia, a semi-arid, hilly to mountainous region which is fairly sparsely populated, but rich in mineral deposits and mining districts. The orebody at Tsumeb outcropped spectacularly (the so-called ‘green hill’) above the largely flat, scrubby semi-desert landscape around it, and was known amongst the local Herero tribes for thousands of years. Small quantities of secondary copper ore, chiefly malachite, were removed by the native tribes and traded to other parts of Namibia, where they were smelted by simple means into metallic copper. The first recorded mention of the deposit by Europeans was in 1857 when missionaries traveling into ‘bushman territory’ of what was then South-West Africa (SWA) from South Africa wrote back to Johannesburg to report the ‘most incredible exposure of colored copper ores (sic) they had ever seen.’

Tsumeb Minerals are Amazing, like this Smithsonite – Click here and find amazing photos
Small-scale mining continued until 1900, when larger industrial development of the deposit began thanks to a new railroad connecting then quite remote Tsumeb to the coast from which ore could be transported to distant refineries. Initial production had to be hauled over 500 km. to Swakopmund, making it economically unfavorable. After SWA became a German colony, a corporation called the Otavi Minen-Und-Eisenbahngesellschaft, or OMEG was formed, which quickly removed the remains of the famous ‘green hill’, expanded it into a modest open-pit mine, and soon went underground, chasing higher and higher grades as the pipe-like orebody continued vertically down. After Namibian independence from Germany, OMEG merged into the Tsumeb Corporation, controlled at various times by Newmont Mining and other large global mining consortiums. The deposit, while not especially large on a global scale, had a unique set of mining challenges including its steeply-dipping pipe-like form and the constant danger of flooding, due to extensive paleo-karst systems housing a large aquifer. High grades (averaging 10% Pb, 4.3% Cu, 3.5% Zn, 100 ppm Ag, 50 ppm Ge), made the tremendous expenditure associated with both dewatering the mine and treating the metallurgically-complex ores profitable (Lombaard et al, 1986). When the great Tsumeb mine closed in 1996 and was allowed to flood, over $5 billion in copper, lead, zinc, silver, germanium, and gold had been produced. An effort by a consortium of mineral collectors and dealers led by Ian Bruce was made in the late 1990’s to re-open part of the Tsumeb mine for mineral specimen mining, but unfortunately this was found to be uneconomic.
Geologically, Tsumeb is part of a small group of high-grade polymetallic, carbonate-hosted ‘ore pipe’ deposits. Mineralization is confined to dolomitic carbonate rocks of the upper Otavi formation, which is part of large supracrustal sequence overlying Precambrian gneiss and granite in northern Namibia (Guilbert & Park 2007). Mineralization occurs in a pipe-like, steeply dipping cylindrical body bounded by ‘psuedoaplite’ dikes, which are probably clastic dikes related to complex salt tectonics and salt diapirism which has been observed elsewhere in this sequence in Namibia and neighboring countries. Karst-dissolution, breccias, collapse structures, and faulting are common, indicating mineralizing fluids were probably fairly robust over an extended period. Ore grades are highly variable, but along intersections between favorable lithology and structure, massive sulfide lenses containing up to 60%(!) combined Cu, Pb, Zn, Ag & Ge were commonly found (Guilbert & Park 2007). The great wealth of collectible minerals at Tsumeb is mainly due to the great depths of oxidation and supergene enrichment of primary sulfide ores, with supergene mineralization predominating above 400 meters depth, and considerable secondary mineralization persisting to depths of 800 meters! This great oxidation depth is probably related to the permeability of the dolomitic carbonate rocks, as well as the unique influence of salt tectonics on mineralization. The unusually chemically-diverse hydrothermal fluids associated with mineralization also introduced many unusual metals such as Ge, Ga, Sb, & As, contributing to the huge list of mineral species found at Tsumeb.
Ask any mineral collector which species he or she first thinks of when the word ‘Tsumeb’ in mentioned, and the answer will invariably be ‘azurite.’ Not only did Tsumeb produce azurite crystals of outstanding color and luster, the size was world-class as well, with specimens such as the ‘Newmont Azurite’ which features crystals to over 20 cm(!). Azurite occurs in a variety of forms, from blocky to tabular to elongate prismatic crystals. Malachite psuedomorphs after azurite are a specialty, with faithfully-preserved sharp green crystals to 10+ cm. being fairly common and occurring in spectacular large groups. Associated minerals include smithsonite, calcite, duftite, olivenite, mottramite, and more, leading to combinations with outstanding colors and aesthetics. Dioptase is probably a close second in terms of beauty and fame from Tsumeb, with the most famous and sought-after specimens consisting of rich ‘carpets’ of brilliant blue-green crystals to 2+ cm. on a matrix of snow-white dolomite or calcite. Thousands of such specimens were found in the 1960-70’s, but are now quite scarce (or expensive) on the collector market.
Cerussite is another mineral which reaches its worldwide zenith at Tsumeb, occurring in a variety of forms and colors, from complexly-crystallized, reticulated ‘snowflake’ crystal groups (sometimes to 30+ cm!) to heart-shaped twins in beautiful limpid shades of yellow to clear. Individual crystals have been reported up to 60 cm(!), surely a record for the species. Inclusions can cause cerussite crystals from Tsumeb to appear green, blue or red, and the luster is typically high. Mimetite, one of the most common secondary minerals in both major oxidation zones at Tsumeb, also reaches a zenith here, with the most famous crystals being from the 1971 ‘gem pocket’ which produced gem-clear yellow crystals to 6 cm., with only a few dozen good specimens being found. A good mimetite from this pocket would today easily set you back several tens of thousands of dollars.
No article on Tsumeb would be complete without mentioning smithsonite, which also probably sets a global standard at Tsumeb. Perhaps most remarkable about Tsumeb smithsonite is the variation in color, spanning colorless to yellow to pink to green to deep-blue, and seemingly every shade between. Tsumeb is also one of the few localities in which smithsonite forms well-developed crystals, often to several cm. each and with outstanding luster. As far as rarer species, it is futile to try and cover the breadth and uniqueness of rare minerals from Tsumeb. Species such as cuproadamite, alamosite, arsentsumebite, bayldonite, leadhillite, ludlockite, and olivenite demonstrate that rare does not always mean ugly. While Tsumeb as a mine is probably closed forever, the good news for collectors is that the veritable flood of specimens during the 20th century means that the average collector should be able to obtain good Tsumeb specimens for many years to come.

Famous ‘Newmont’ Azurite; Tsumeb Mine- approximately 30 cm. across with crystals to 15 cm. (photo © American Museum of Natural History)
5.) Kola Peninsula, Russia

Workings of the Umbozero Mine, Khibiny Massif: K. Dembicz photo/© Spirifer Minerals 2009
Locality number 5 risks taking us back into the realm of ‘ugly and rare’ after letting our imaginations roam the colorful crystalline landscape of Tsumeb. But wait! Have you seen the starburst-like sprays of golden astrophyllite crystals in a snow-white matrix? The gemmy, sword-like natrolite crystals to 30 cm? The delicate pink tugtupite, hackmanite, and ussingite whose neon expanses hide dozens of minerals known nowhere else in the world? The Kola Peninsula, extending into the Arctic region of northwestern Russia to the east of the Scandinavian Peninsula, is truly a world-class mineral locality and geological treasure house. While I am deviating somewhat from the ‘single locality model’ in including the Kola Peninsula (an almost 100,000 square kilometer area), I think this inclusion is justified in that the main mineralized areas, the Khibiny and Lovovero Massifs, are in fairly close proximity to each other, and show strong geological, temporal, and mineralogical similarities.
The Kola Peninsula (taken here to include to massifs of Khibiny & Lovozero) were long shrouded in mystery to western collectors and mineralogists, and for many still conjure images of distant snow-covered slopes and small ‘rocks’ with arrows on them. Part of this mystery is political, as the region is in a remote part of what was up until 1994 the Soviet Union, and the strategic nature of the mineral deposits at Kola as well as Cold War tensions limited communication with outsiders about the treasures of the region. Inside Russia (and the USSR), however, is a rich history of geologic exploration at Kola. While native peoples of the Inuit culture made Kola home for thousands of years and surely took note of the unusual appearance of many of the rocks there, ‘modern’ exploration began in earnest in the 1920’s with the discovery of massive deposits of magmatic apatite at Lovozero (Pekov 2000). Since the USSR had limited ability to import outside resources and did not have large known ‘conventional’ phosphate ores, the rather unusual initiative was taken to begin mining these apatite ores for their phosphorous content, a critical ingredient in the ‘state farm collective’ program of industrial agriculture.

‘Stars’ of Lamprophyllite in Nepheline, Umbozero Mine: K. Dembicz photo/© Spirifer Minerals 2009
In the 1930’s, as the apatite mines were developed, leading Soviet mineralogist and geochemist Alexander Fersman visited the area and took note of the mineral eudialyte, a complex silicate enriched in Zr and REE’s. Loparite, another complex REE species, was discovered in 1934, and soon an outpost of the USSR Academy of sciences was established at Khibiny (Pekov 2000). Loparite generated much interest both to scientists and the government, as it was a potentially-economic ore of the strategic metal niobium, as well as other rare metals. In fact, much of the development of the rare metal industry in the USSR was shrouded in secrecy, even to those involved in it. Exploration and mining in Kola ceased temporarily during WWII, when many of the mills and mining operations were converted in munitions plants and military equipment factories. Mining and exploration resumed after 1945, but this period leading up to Stalin’s death was somewhat of a dark period for Kola, as most labor at the mines was in the form of ‘gulag’ prisoners, many there for political reasons. Nonetheless, scientific inquiry into the unique geology of the region continued, with the publication of ‘Petrology of the Lovozero Massif’ in 1972 by Bussen & Sahkarov marking a research milestone (Pekov 2000).
Exploitation of both apatite and rare metal ores continued at a fairly steady pace through the early 1990’s, when democratization of the country and the fall of the USSR brought a slowdown in heavy industry, which has continued until today is some areas. Research on the peninsula, however, continued to be strong, with many papers and significant discoveries by scientists such as A.P. Khomyakov, I. Pevok, P. Kartashov, and others. Apatite mining at the Kirovskii mine on the Khibiny Massif and several locations on Lovozero continues today, but unfortunately many rare metal operations such as the famed loparite deposit at Umbozero have fallen in disrepair. Hopefully, with economic strengthening of Russia and renewed global interest in rare metals, particularly the largely-untapped eudialyte ores, mining will return full-force to the region, and with it new mineralogical treasures to be found.

The great Russian mineralogist A.P. Khomyakov in his laboratory in Khibiny in 1992, with list of possible new minerals behind him (photo © O.T. Ljostad/mindat.org)
Geologically, Khibiny and Lovozero are quite complex, but can be explain most simply as a pair of complex multiphase alkalic igneous intrusions of Devonian age, each with a surface expression of ~600 km2, which have intruded the Archean crystalline rocks of the Baltic Shield (Pekov 2000). The intrusions are in deep graben-like structures, likely related to syn-intrusive rifting, and are partly filled with middle Paleozoic age volcanic and volcanoclastic rocks. The main rock types in the massifs are nepheline syenites and related silica-undersatured, nepheline and sodalite-normative alkaline igneous rocks, but there is also a complex suite of mafic rocks ranging from trachyte to phonolite to gabbro, an important piece of evidence in the interpretation that alkalic intrusions often have genetic links to primitive mantle-derived mafic magmas in intraplate settings (Pekov 2000). Enrichment in phosphorous of one of these magmatic pulses led to the development of an immiscible, possibly late-stage melt from which large quantities of primary magmatic apatite were about to crystallize (Pekov 2000). The diversity and rarity of the rock types encountered at Lovozero and Khibiny indeed led to a whole new nomenclature for such rocks- urtites, lujavrites, foyaite, riscchorites and malignites are all important and rare alkalic rocks at Kola. The multiphase and chemically-variable nature of the intrusions created spectacular layering and zonation, both vertically and in cross-section, of different rock types. Both intrusions included eruptive phases and concordantly shallow intrusive units showing spectacular poikilitic (sieve-like mineral intergrowth) textures and evidence of subsolidus crystallization (Pekov 2000). With subsolidus (below the normal melting curve of that mineral assemblage) crystallization came widespread metasomatic alteration, in which one element is removed and replaced by a different element, typically by hydrothermal fluids. Metasomatism and related pegmatite formation are undoubtedly the main factors in establishing the incredible mineralogical diversity of Khibiny & Kola. Pegmatites are abundant in numerous rock types of both massifs, and while often small (a large pegmatite might be ~20 x 1.5 m), they can contain an amazing abundance of minerals: one pegmatite encountered underground in the Umbozero loparite mine contained over 80 minerals! Finally, the sub-Arctic setting of the massifs and lack of appreciable vegetation combined with extensive glacier scouring and erosion means that exposure is generally excellent, and many new pegmatites and mineral localities are discovered each field season.

Loparite-(Ce) twins of matrix to 1 cm: Khibiny. (photo © B. Kantor/mindat.org)
Mineralogically, 507(!) different mineral species have been reported from Khibiny, and 376 from Lovozero. Truthfully, both massifs include dozens of individual localities, but they generally share such strong genetic and chemical characteristics that for the sake of discussion they will be referred to as a single locality here. Starting on the aesthetic end of the mineral spectrum, many collectors will be surprised to learn that both Khibiny and Lovozero have produced spectacular, beautiful crystallized specimens of numerous minerals. Perhaps best-known is astrophyllite, a complex Na-Ti silicate which occurs as golden sprays of radiating crystals to 10+ cm in snow-white matrix. Eudialyte, a rare species which is sometimes a rock forming mineral(!) at both massifs, rarely occurs as sharp, bright pink to red crystals to 5+ cm. Natrolite, a common mineral both in the more evolved rock units and the pegmatite’s of Kola, occurs as sprays of gemmy, sharp clear crystals up to an impressive 30 cm. Loparite, the important ore of Nb & REE’s, occurs as sharp black cubic crystals to several cm, sometimes in attractive interpenetration twins. Neptunite and Lorenzenite, two chemically-different but somewhat similar looking minerals, occur in attractive dark red to black crystals up to the size of a finger. Elpidite, the rare Be silicate, occurs as large hand-sized sprays of radiating white crystals. Kovdorskite, a rare Mg phosphate from the Kovdor apatite mine, occurs as beautiful clear crystals to several cm. The rare fluoride Villiaumite (NaF) rarely occurs as euhedral crystals, but forms attractive ‘nests’ of bright red cubic cleavages to 20+ cm in some pegmatites.
In terms of ‘rare’ species (rare being a relative term at Kola), there are hundreds of minerals, and a monograph could (and has) been written on them. Suffice it to say that most occur as attractive crystals <1 cm., while others are only massive, but many, massive or otherwise, have beautiful color and texture, which renders them desirable collection specimens nonetheless. Pegmatite such as the Shkatulka pegmatite in the Umbozero Mine and the pegmatites of Kukisvumchorr Mountain at Khibiny are world-renowned for their complexity. Many are beautiful even to the lay-person, presenting coarse-grained intergrowths of red eudialyte, green amazonite, giant microcline and nepheline crystals, chlakovite, ussingite, and tuptupite and delicates shades of crimson and pink, and smaller ‘nests’ of vibrantly colored REE and rare metal-bearing minerals. Given that most of these pegmatite’s were discovered as surface outcrops and have never been mined, the treasures of Khibiny & Lovozero should be accessible for generations of mineralogists to come.
6.) Illinois-Kentucky Fluorspar District, USA

Galena on Fluorite: Denton Mine, Harris Creek Sub-District, Hardin Co. Illinois (photo © MIM Museum, Beirut)
In light of many of the preceding localities such Mont Saint Hilaire and Tsumeb whose world-class status is indisputable, I was a little hesitant to include the Illinois-Kentucky Fluorspar District in the Midwestern USA to the ‘top ten’ list, but as much as I tried to ignore it, it kept coming back into my mind. While certainly not as diverse as Mont Saint Hilaire or unique as Tsumeb, the fluorite mines of Southern Illinois and northern Kentucky constitute one of the earth’s premier endowments in beautiful, crystallized mineral specimens. During their operation from the early 19th century up until 1996, literally tens of thousands of fine specimens of fluorite, calcite, sphalerite, galena, witherite, and more were saved, and there is scarcely a serious mineral collector in the world who does not own a specimen from the district. Combine this abundance of specimens with a seemingly endless variety in color and form in this (albeit limited) species list and you have the makings of a world-class locality.

Fluorite: Minerva #1 Mine, Cave-in-Rock, Illinois- 7 cm. across (photo © James Elliot/FMI: now in the MIM Museum, Beirut)
The history of mining and minerals from the Illinois-Kentucky fluorspar district is closely linked to the history of the Midwestern US and the westward migration of pioneers and prospectors in the 18th and 19th centuries. In the early 19th century, Southern Illinois was still a fairly wild and undeveloped region, with local Indian tribes outnumbering white settlers. This changed as word of rich outcropping of galena ore (associated with then less-valuable fluorite) were found along the banks of the Ohio river near what is now Cave-in-Rock, and prospectors as well as farmers began settling the area. Mining in the 19th century focused mainly on galena/sphalerite Pb-Zn ores, and it was not until a steelmaking process in the 1880’s required fluorite for flux that mining in the district shifted to the massive fluorite (or ‘fluorspar’) deposits (Goldstein 1997). Before WWII, most mining was focused on the Rosiclare area, but this moved to Cave-in-Rock in later years, with large underground mines such as the Minerva #1, Denton, and Annabel-Lee accounting for most of the fluorite production in the later 20th century. Goldstein (1997) noted over 95 individual mines in the Illinois side of the district, and over 130 on the Kentucky side, though to be certain many of these are small prospects, and a handful of large mines on the Illinois side accounted for 75% of modern production. The district supplied over 90% of the US fluorite production, and large amounts of lead, zinc and barium were also recovered (Goldstein 1997). Unfortunately while the huge fluorite reserves in the district are probably far from depleted, rising production costs and cheap imported Chinese fluorite made mining economically unfeasible in the late 1990’s, and the last mine, the Annabel Lee, closed in 1996, marking the end of over 200 years of fairly continuous mining in the Illinois-Kentucky Fluorspar District.
Geologically, the Illinois-Kentucky fluorspar district seems deceptively simple, but in reality is part of a complex and still-poorly understood region which has been affected by processes ranging from faulting to sedimentation to unusual igneous intrusions. The surface and near-surface (upper few kilometer) geology is dominated by sedimentary rocks ranging from middle Devonian to early Pennsylvanian in age (Goldstein 1997). The fluorite-barite-galena-sphalerite orebodies occur as two generalized types, bedding-replacement deposits which are mainly horizontal and controlled by stratigraphy, and steeply-dipping veins which follow structures and can extend to great depths. Really, these two ore deposit styles are probably related, as the ‘bedding replacement’ deposits require a structural conduit for mineralizing fluids to reach a favorable limestone bed where replacement can occur. Both orebody types occur in a large anticlinal structure known as Hick’s Dome, whose uplift is probably related to regional compression as well as emplacement of a potential (never interested by drilling) deep alkalic intrusion, which may have been the source for fluorine for the deposits. The Illinois-Kentucky fluorspar district sits in the most heavily faulted area of the Midwestern USA, and these faults provided the ‘structural plumbing’ necessary for creation of the numerous ore deposits. The ‘smoking gun’ showing genetic connection between alkalic intrusive rocks and the fluorite deposits would probably involve isotope and trace element geochemical work, testable by modern methods, but little serious research on the fluorite deposits has occurred since mining ceased in 1996. Current literature suggests that locally-derived brines (salty fluids) from probably evaporitic beds within the limestone package mixed with magmatically-derived fluids, which may have triggered mineralization and precipitation of fluorite (Grogan & Bradbury 1967). Whatever the case, the combination of large, extensively-mineralized areas and a propensity for brecciation and attendant open pocket formation in the district proved to be a bonanza for mineral collectors.
Without a doubt, the premier mineral from the Illinois-Kentucky fluorspar district is, as the name suggests, fluorite. Fluorite from the district probably shows more variation in color than at any other locality, ranging from purple to blue to yellow to gray to pinkish and every shade in-between. The only ‘dominant color’ missing is the rich greens of the North Pennines Ore Field fluorites from the UK, though green crystals were found rarely in several of the older Illinois mines. Perhaps most famous and coveted are the large, gemmy groups of cubic crystals showing crisp color zonation, typically yellow with violet edges or vice-versa, from mines such as the Annabel-Lee, Minerva #1, and Denton. Most of these came out from ~1980-1995, and pockets were often so abundant during this period that high-quality fluorite sold either by the pound or by ‘the table’ at shows or at a miner’s residence. Competition for top specimens was fierce, however, and prominent dealers in the region such as Ross Lillie, Dan Weinrich and Mark & Joe Kielbaso have many stories about racing down to Cave-in-Rock or Rosiclare to see ‘the next big find’ moments ahead of their competition (Goldstein 1997).
The early years (~1900-1950) of fluorite production included important specimen-producers such as the W.L. Davis-Deardorff mine, which produced delicate violet fluorite crystals on a distinctive drusy quartz matrix, and the Hill-Ledford, which produced some of the largest single fluorite crystals in the district, up to 45 cm across! Sadly, as they had little value at the time due to their perceived commonness, many were either damaged upon removal, or broken down into ‘spar octahedrons’ which are produced by exploiting fluorite’s two perfect cleavage directions with a small hammer. During this period, many fine examples of galena and sphalerite were also found, with galena crystals from the Hill-Ledford mine sometimes reaching 15 cm on edge. The Minerva #1 mine, discovered somewhat accidentally when a night-shift driller salted their drill hole cuttings to hide their nighttime work absences, became the premier locality for the rare barium minerals witherite and benstonite from the late 1940’s up to the 1980’s (Goldstein 1997). Witherite occurs there as sharp white to yellow barrel-shaped crystals up to 15 cm, sometimes aesthetically isolated on fluorite or barite matrix.

Witherite: Minerva #1 Mine, Cave-in-Rock, Hardin County, Illinois, 7.5 cm (photo © Joe Budd/irocks.com)
Barite is another fairly ubiquitous species from the Illinois-Kentucky fluorspar district, with the best crystals coming from the Minerva #1 and Denton mines, often associated with colorful fluorite and calcite. In the 80’s and early 90’s, the Denton and Annabel-Lee mines amazed the world with their brilliantly lustrous, golden-yellow calcite crystals, often as large twins on matrix. Celestine, a somewhat rare mineral for the district, was found as excellent blue-gray crystals to 5 cm on fluorite from the Annabel-Lee mine (Goldstein 1997). Galena, having previously consisted of sharp but somewhat dull crystals from the W.L. Davis-Deardorff and Hill-Ledford mines, was found as brilliant cubo-octahedrons on purple fluorite at the Denton mine. Many fluorite crystals show fascinating dissolution textures, where later fluids have corroded them into bizarre shapes and sometimes deposited new minerals, such as paralstonite and smithsonite. Strontianite in attractive yellow to white sprays was sometimes associated with these altered fluorite crystals as well, and occasionally, perfectly circular ‘holes’ would be seen in otherwise unaltered fluorite crystals where inclusions of spherical barite had dissolved, sometimes called ‘drillholes’ by the miners. While current economic conditions are not bright for the return of fluorite mining to the Illinois-Kentucky fluorspar district, the good news is that the sheer number of specimens produced means that every collector can own a piece of this world-class mineral locality for many years to come.
7.) Bisbee, Cochise County, Arizona USA

Bisbee Masonic Lodge holding a 1887 meeting in a huge ‘cave’ in the Copper Queen Mine, Bisbee, Arizona (photo © Library of Congress)
Bisbee, situated in the rolling scrub oak hills of far southern Arizona just north of the Mexican border, is a world-class mineral locality in every sense of the word. From the late 1870’s up until 1975, Bisbee produced 3.6 million tons of copper, 161,000 tons of zinc, 147,000 tons of lead, 100 million ounces of silver, and 2.7 million ounces of gold, making it one of the richest mining districts in the world for its size (Graeme 1981). Bisbee’s lasting legacy, however, will probably have more to do with the thousands of fine and colorful mineral specimens it produced than metal statistics. Collections all over the world include crystals of azurite, malachite, native copper, calcite, and more from Bisbee mines. Additionally, while mining for copper at other metals no longer takes place at Bisbee and the community has embraced a more artistic side and tourism, research on the ore deposits is ongoing and many new species, both to the district and to science have been discovered since mining stopped.

Cuprite (2.5 cm. crystal) on malachite from the Southwest Mine (specimen and photo © Richard Graham)
The mining history of Bisbee is fairly recent, beginning in the late 1870’s when prospectors Jack Dunn and George Warren visited the area from nearby Tombstone, a recently-established silver camp, and noted abundant outcrops of colorful gossan, a good indicator of mineralization at depth (Graeme 2008). They worked the near-surface ores over the next several years, ad eventually the Copper Queen mine was developed in the early 1880’s. The Copper Queen, a series of high-grade supergene orebodies in Naco limestone, featured not only high copper grades but spectacular ‘caves’ lined with stalactites of azurite, malachite, and other secondary copper minerals. Thanks to the foresight of these early miners, many of these specimens were preserved in prominent East Coast museum collections, but many more were destroyed and melted down for their copper content during mining. Mining accelerated in the 1880’s and 90’s as additional high-grade, near-surface copper orebodies were discovered, and Bisbee quickly grew into a typical frontier ‘boom town’, with businesses crowded into the narrow Brewery Gulch downtown.

Azurite with Malachite: Czar Mine (14.5 cm across; specimen and photo © Joe Budd & irocks.com)
James Douglas started the Phelps & Dodge Mercantile company, which acquired claims adjacent to the Copper Queen which ended up propelling the company into one of the world’s largest copper producers of the 20th century, the famous Phelps Dodge Corporation. The Copper Queen mine operated independently by the Copper Queen Mining Company, but after ~1920, pressures to consolidate meant that the mines were acquired by Phelps Dodge, which ruled mining in Bisbee for the next 50+ years (Graeme 2007). Mines such as the Czar, Holbrook, Copper Queen, Southwest, and Junction mined fabulously rich and extensive copper ores, with significant recovery of Au, Ag, Pb, & Zn as well. Miners were long aware of the value and beauty of good mineral specimens- many anecdotes exist about miners trading fine minerals for haircuts, drinks, and often cash from visiting mineral dealers and museum curators (Graeme 2008). The final chapter of mining at Bisbee involved the type of large tonnage, low-grade open pit mining now favored in Arizona, focused on the porphyry from which most of the high-grade veins and carbonate-replacement deposits originated. This mine, the Lavender Pit, is still quite visible today, dominating the south end of Bisbee. Relatively small-scale mining of some remaining high grade portions of orebodies in the Junction and Holbrook mines continued into the early 1970’s as well, but these closed eventually due to rising costs and lower yield (Graeme 2008).

Spinel-twinned group of Native Copper Crystals: Bisbee, Arizona (5.3 cm, specimen and photo © Joe Budd & irocks.com)
The geology of Bisbee has been studied in detail since Frederick Ransome of the U.S. Geological Survey published his landmark monograph in 1904. Bisbee, like much of southern Arizona, in underlain by Precambrian schist and quartzite of the Pinal group, which in turn is overlain but 1600-2000 meters of mostly Paleozoic sedimentary rocks, dominantly limestone (Graeme 2008). Beginning in the Jurassic period ~180 million years ago, igneous activity and associated faulting introduced massive amounts of pyrite to the Bisbee area, which replaced selective limestone beds and also was associated with minor copper, silver and gold (Anthony et al. 1995). Later, during regional extension associated with basin-and-range tectonics across southern Arizona in the Cretaceous to Eocene periods, many large copper-bearing porphyry intrusions were emplaced, including Ray, Morenci and Bagdad. At Bisbee, however, a similar copper porphyry, the Sacramento stock was emplaced much earlier, around 104 million years ago, and high-grade vein deposits were probably concurrent with this intrusion (Bain 1952, Anthony et al. 1995). Lead-zinc deposits of the carbonate-replacement type were formed during this event, and finally, somewhat later, a third major pulse of mineralization overprinted some earlier Cu-Pb-Zn deposits, enriching them in unusual metals such as tin, bismuth, tungsten, tellurium, and antimony (Graeme 2008). Uplift and erosion during the more recent Eocene period led to the devlopment of extensive supergene enrichment zones in many deposits, in which most of the colorful and well-crystallized minerals reside. It is this complex and multi-stage geologic history that gave Bisbee its unique mineralogy.
The mineralogy of Bisbee is rich and varied, with over 322 mineral species reported (Graeme 2008). The ores are often highly complex, with assemblages of rare tin, tungsten, and tellurium minerals that are just beginning to be understood. Of course, the stars of Bisbee are azurite and malachite, both occurring in quantities large enough to be considered major ores in the early years. Crystals of azurite from Bisbee occur in a variety of forms, from classic ‘rosettes’ of flattened blocky crystals, to elegant prismatic blades to 10+ cm on matrix. Tsumeb and the new Milpillas mine not far from Bisbee may be close competitors for the title of ‘world’s best azurite’, but Bisbee can hold its own, specimen-for-specimen, with most anything from these locales. Malachite often replaces azurite at Bisbee, and sharp psuedomorphs in clusters to 20+ cm are a specialty. Cuprite is another species for which Bisbee is world-renowned, with the best specimen consisting of 2.5 cm, lustrous, gem-red crystals on malachite; truly a spectacular piece. ‘Chalcotrichite’, or fibrous nests of cuprite needles, is common, often associated with native copper. Native copper was locally abundant at numerous levels in the major mines, and fine crystals, often showing spinel-law twinning, occur in hand to basketball-sized groups, giving the Michigan Copper Country a ‘run for its money.’ Calcite is a particularly varied and beautiful Bisbee mineral, often colored by inclusions of various copper minerals, resulting in bright red or green to blue groups. The ‘caves’ of the early supergene enrichment zones contained huge quantities of ‘floss ferri’ aragonite and delicate groups of calcite crystals showing stalactitic growth features.

Spangolite with cuprite: Czar Mine, Bisbee, Arizona: crystals to 1.7 cm (photo & specimen © Harvard University)
Bisbee is the type locality for 7 species, including the rare and beautiful copper minerals Spangolite and Paramelaconite, both highly prized today. Shattuckite, another rare copper mineral, was also discovered at Bisbee. Turquoise, while not associated with historic mining at Bisbee, was found in considerable quantity and quality in more recent years adjacent to the Lavender open pit. Paratacamite, Covellite, Conichalcite, Claringbullite, Conellite, Chalcoalumite (TL), and brochantite are colorful and reasonably abundant at Bisbee, despite being globally rare copper minerals. Recent research on Bisbee has added numerous new species to the list, and the previously ignored ‘massive sulfide ores’ of deep mines like the Campbell and Holbrook are now recognized as having very unusual geochemical signatures, with strong enrichment in indium, gallium, tungsten, tin, and tellurium, suggesting the genesis of the deposits likely involved multiple geochemically unusual hydrothermal fluids. Phelps Dodge successor Freeport-McMoran has been considering re-opening and expansion of the Lavender pit in Bisbee in recent years, so when copper prices improve, we may once again see mining return to the great town of Bisbee (Jaworski pers. comm. 2014).
8.) The Ojuela Mine, Mapimi, Durango Mexico

Famous Roebling Suspension Bridge at Ojuela (photo © mexconnect.com)
Mention the Ojuela (pronounced O-whale-ah) mine to collectors and visions of vibrant green Adamite pinwheels, neon yellow Legrandite sprays, and rich orange Wulfenite groups are sure to be conjured, or perhaps the 310 meter length of the famous Roebling suspension bridge, the longest in Latin America, contrasting with rugged desert landscapes. The Ojuela mine, located in the state of Chihuahua close to its border with Durango in north-central Mexico, is home to over 137 mineral species, with 7 of them having their type locality there. Over 6 million kilograms of silver and 49,000 kilograms of gold were produced from Ojuela, in addition to substantial lead and zinc, totally in value to over 2 billion dollars in nearly 400 years of production (Panczner 1987). Its history is rich as well, from the discovery of the deposits by Spanish Jesuit priests in 1598 up until today, where mining, mainly for mineral specimens, is ongoing (Haghenbeck & Haghenbeck 2011).
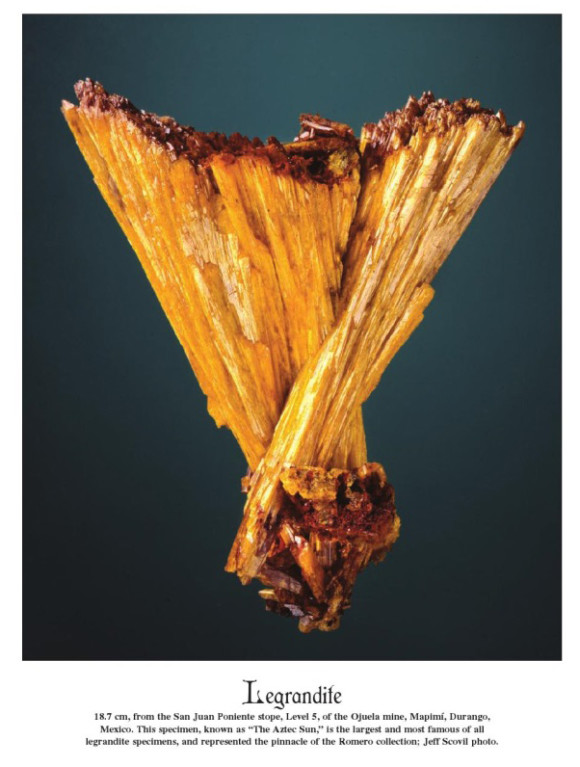
Famous ‘Aztec Sun’ Legrandite; ~20 cm, Ojuela Mine (photo © Jeff Scovil & the Mineralogical Record)
Mining for silver began in the early 17th century and continued at a small scale before accelerated in the mid-19th century with the importation of modern mechanized mining methods from Europe and the USA. To solve the conundrum of connecting mining operations with the town located across a deep, rocky gorge, a huge pedestrian suspension bridge measuring 310 meters across was constructed in 1898 by John Roebling & Sons (Haghenbeck & Haghenbeck 2011). John Roebling’s son, Washington Roebling, was a leading mineral collector of his era, so the improvement of Ojuela, already then known for fine minerals, must have been of particular satisfaction to him. Mining at Ojuela continued to grow up until the Mexican revolution in 1910, when the country was swept into chaos and Penoles, the large mining conglomerate in charge of operations, was nationalized (Haghenbeck & Haghenbeck 2011). After the revolution, mining continued, eventually creating over 450 kilometers (!) of underground workings. Large-scale mining ceased in 1945, but since that time, small groups of miners organized under collectives or cooperatives have produced a mix of silver-polymetallic ore and mineral specimens. Mineralogists of the early 20th century such as W.F. Foshag and Dan Mayers helped popularize the unusual and colorful minerals of Ojuela, and miners found that they could make better wages collecting crystallized minerals than mining ore, a tradition that has continued up until today.

Adamite ‘pinwheels’ on matrix to 4 cm from the Ojuela Mine (photo © Jeff Scovil)
The geology of the Ojuela Mine and the Mapimi region is dominated today by typical basin-and-range topography and structure, with similar mountain ranges, valleys and landscapes to Southern Arizona or New Mexico. Precambrian age granite and schist is overlain by a thick sedimentary sequence, dominated at Ojuela by Cretaceous limestones and dolomites (Panczner 1987). The Ojuela mine exploits not one but seven different pipe-like orebodies, called ‘chimneys’, and associated ‘mantos’, or carbonate-replacement orebodies controlled mainly by stratigraphy. These chimneys and manto deposits extend to depths of over 900 meters, with oxidation occurring at depths of up to 500 meters (Panczner 1987). Similar to Tsumeb, this great depth of oxidation and supergene enrichment couples with ‘receptive’ carbonate host rocks are largely responsible for Ojuela’s great mineralogical diversity. 4 main mineralization styles occur at Ojuela: copper-enriched ‘contact’ ores, lead-zinc ores, silver-lead ores, and the ‘barren’ carbonate zone (Panczner 1987). Each of these zones contains different minerals assemblages.
Mineralogically, the Ojuela mine is perhaps most famous for its arsenate (arsenic-containing) secondary minerals, such as adamite, legrandite, and kottigite. Adamite, perhaps the most famous mineral from Ojuela, occurs as spectacular ‘pinwheels’ on vibrant green crystals to several cm each, as well as sharp individual crystals, typically on an aesthetically-contrasting gossan matrix. Cuprian adamite is relatively abundant at Ojuela and has a distinct blue-green shade different than copper-free adamite. The most desirable, however, is the manganoan adamite, colored purple and occurring as vivid bundles of crystals to 5+ cm on matrix. These purple adamites caused quite a stir in the mineral world when a major pocket was discovered in 1981, and Texas oil man Perkins Sams spent many thousands of dollars of several top examples now in the Houston Museum of Nature & Science. While adamite is still being found at Ojuela, most modern production consists of small crystals in vugs in matrix, and it appears the often very large (and very inexpensive by modern standards), plates of pinwheels or bladed aggregates of crystals are a thing of the past.

Wulfenite on Mimetite: Ojuela Mine (7 cm, specimen and photo © Joe Budd & irocks.com)
A close second to adamite from Ojuela is legrandite, another rare zinc arsenate species which reaches its zenith at Ojuela. Probably the most famous specimen, consisting of an aesthetic ‘V’ pair of sharp yellow crystals to 20 cm(!) is known as the ‘Aztec sun’ and for many years was the centerpiece of the Dr. Miguel Romero collection (it is now in the MIM museum in Beirut). Its sale and the extraordinary price (rumored to be around $2 million USD) associated with it also became a talking point in the mineral community, and in a curious case of ‘trickle down economics’, all other legrandites, even thumbnails, seemed to see a concordant increase in their price tags. Nonetheless, enough legrandite was produced that the average collector can still acquire a modest example of this beautiful and classic species from the Ojuela Mine.
Other rare and beautiful arsenate species from Ojuela include brilliant green Austinite crystals, blue Kottigite sprays, and blue-gray Symplesite blades. Equally famous (and much more abundant today) are specimens of green, botryoidal mimetite hosting lustrous orange wulfenite crystals, up to several cm in size. Wulfenite from Ojuela is somewhat unusual in its variety of crystal forms: almost equant, pseudo-cubic crystals are not uncommon, as are elongated, dipyramidal crystals. In recent years, many excellent, very aesthetic examples of this combination have been found. Hemimorphite is another attractive zinc mineral which is abundant at Ojuela- groups of parallel white prisms forming an almost botryoidal ‘carpet’ on gossan matrix have been found up to 40+ cm. Several minerals also noted from Tsumeb occur as attractive examples from Ojuela, such as bayldonite, tsumcorite, and duftite. Additionally, Ojuela is the type locale for a number of species, including the rare arsenates lotharmeyerite, mapimite, ojuelite, and miguelromeroite. Continued mining by cooperatives and independent specimen diggers mean that good minerals and perhaps new species will be found at Ojuela for many years to come.
9.) San Diego County, California Gem Pegmatites

Famous 25 cm-wide ‘cadelabra’ blue-capped Elbaite Tourmaline from the 1972 Tourmaline Queen Mine find (photo © Harold & Erika Van Pelt)
Gem pegmatite districts, typically of the Lithium-Tantalum-Cesium (LCT) pegmatite family, are not especially rare on earth, with good examples occurring on most continents, including the mountains of Pakistan/Afghanistan, Minas Gerais State in Brazil, and Oxford County Maine, USA. What makes the pegmatites of Southern California special however, is their concentration, both of individual pegmatites and of collectible (and often beautiful) mineral species. While gemmy, polychromatic crystals of tourmaline, spodumene or beryl first come to mind when San Diego County in invoked, the district is also an important source of fine crystallized examples of rarer species such as stibiotantalite, rynersonite, hydroxlherderite, and hambergite. Mining for gemstones and mineral specimens has enjoyed an almost 125 year history, continuing today, and interest and appreciation for the minerals of the region, particularly tourmaline, are probably at an all-time high today.

George Kunz examining crystals of spodumene var. Kunzite around 1905.

A large kunzite crystal in the Harvard collection from the same find (both photos © Bill Larson/palaminerals.com)
The rolling, Mediterranean-like hills around Mesa Grande and Pala were quite pastoral in the late 19th century, with farming and ranching occurring around scattered small villages. In 1898, Mesa Grande local Gail Lewis discovered small crystals of bicolor tourmaline loose on the surface of what would become known as the Himalaya pegmatite, and filed a claim on it. Given the strong market for US gemstones at the time, the chief promoter of this market, George Kunz of the Tiffany company of New York, soon got word of the new California tourmaline discovery, and sent secret company agents to investigate the potential for a new gem district (Fischer 2008). Kunz’s men soon began mining tourmaline and other pegmatite minerals at the Himalaya and nearby pegmatites on their own, with most good colored tourmaline going to China for carving into ornamental snuff bottles and other items which were then very much in demand there (Fischer 2008). In 1902, the pegmatites around Pala were discovered, and soon after, a number of crystals of bright pink spodumene were found, to be named ‘kunzite’ in honor of George Kunz. After the Chinese revolution in 1912, demand for carving-grade tourmaline plummeted, as did gem demand in general in the US around the world war periods (Fischer 2008). It was not until the 1950’s, as mineral collecting became increasingly popular in the US along with demand for colored gems that pegmatite mining resumed in San Diego and Riverside counties. Several spectacular discoveries in the 1970’s and 80’s, most notably the 72 find of huge blue-capped pink tourmaline crystals at the Tourmaline Queen Mine. Mines such as the Stewart, Tourmaline King & Queen, Himalaya, and Little Three enjoyed fairly frequent pocket discoveries throughout the middle 20th century, and some are still operating today, though generally on a ‘hobbyist’ or ‘weekender’ level by collectors with other primary forms of employment. Indeed, the challenges of pegmatite mining are perhaps best exemplified by the months leading up to the great 1972 ‘blue cap pocket’ at the Tourmaline Queen Mine, in which miner John McLean and others tunneled over 6 meters through hard, unmineralized, barren pegmatite only to change course slightly into a world-class crystal pocket (Larson 2008). Still though, the search for gems has inspired many a miner and collector, and this search continues today at mines such as the Oceanside and Cryo-Genie.

Stibiotantalite crystals on Rubellite Tourmaline (2.6 cm across), Himalaya Mine (specimen and photo © irocks.com)
Geologically, the gem pegmatites of San Diego County share many similarities with other great gem pegmatite provinces of the world. They are of the lithium-tantalum-cesium (LCT) geochemical family, which are highly evolved pegmatites characterized by stong enrichment in large ion lithophile (LILE) elements such as lithium, cesium, rubidium. They also contain significant boron, fluorine and beryllium, important elements for the formation of tourmaline, topaz, and beryl. Additionally, many pegmatites can be subdivided into rare element (containing minerals such as stibiotantalite and samarskite-(Y)), and phosphate (containing large ‘nodules’ of various phosphate minerals) affinities. In San Diego County, these pegmatites have intruded Cretaceous-age granite plutons of the Peninsular range batholith (Fischer 2008). Pegmatites have been dated at ages of ~93-98 million years, while the main host granite is ~101 million years old (Fischer 2008). This age relationship is typical for highly evolved gem pocket-bearing LCT pegmatites, which form as residual granite melt becomes increasingly enriched in incompatible elements, water, and other volatiles and eventually intrude surrounding, already cooled rock to form much coarser-grained, chemically-complex dikes. Many of the gem-producing pegmatite’s of San Diego County extend along their strike for 100’s of meters, but are only 1-2 meters thick, with spectacular mineral zonation. Unidirectional solidification textures (formed as undercooled magma crystallizes against cold wallrocks) show large euhedral crystals which point inward perpendicularly from pegmatite walls, and at their terminations, a core zone enriched in massive lepidolite, tourmaline and other rarer species may sometimes open up into the much-coveted miarolitic gem crystal pocket. While many pegmatites are strongly deformed by subsequent uplift and faulting, the arid climate combined with fairly near-surface (<5 km) emplacement of the pegmatites means that exposure is excellent, and almost all pegmatites known today were discovered from surface outcrops.
Mineralogically, the San Diego County pegmatite district is fairly diverse, with over 120 minerals known from the pegmatites. The most famous mineral from San Diego County is probably tourmaline, or more specifically the lithium-bearing tourmaline species elbaite. Elbaite occurs in virtually all shades, from hot pink to deep blue to emerald green to nearly jet-black. Probably the most famous tourmaline crystals known today (we will avoid thinking about the many thousands of fine crystals which were destroyed by carving demand by the Chinese in the late 19th and early 20th centuries) are the large (up to ‘beer can’ size!) blue-capped rubellite crystals found in 1971 & 1972 at the Tourmaline Queen Mine. The nearby Tourmaline King pegmatite also produced outstanding, sometimes very large ‘watermelon’ and green-capped pink crystals during this era, sometimes in clusters up to 30 cm. The famous 28 cm-high ‘steamboat’ specimen on display at the Smithsonian is one such example. The Stewart mine produced thousands of elongated rubellite crystals with distinctive ‘hot pink’ color, and similar crystals were found at the Pala Chief mine (Fischer 2008). In the 1980’s and 90’s, many fine elbaite crystals showing horizontal color bands of blue, green and pink were found at the Himalaya mine. Recently, large sprays of similarly color-zoned crystals with curious ‘tapered’ habit have been found at the Cryo-Genie mine. Fine tourmaline continues to be found today in San Diego County.
Next in fame to tourmaline is probably the beautiful pink variety of spodumene, kunzite, first discovered in the Pala Chief mine and nearby Katerina and Vanderberg pegmatites in the early 20th century. Kunzite occurs as flattened tabular, gem-clear pink crystals up to 28 x 15 cm! Indeed, the largest known Southern California kunzite was actually just discovered in 2010 at the Oceanview pegmatite. Beryl also occurs in numerous varieties from heliodor (yellow-green) to goshenite (clear), but probably the most famous crystals are the sharp, gemmy flattened hexagonal pink morganite crystals from pegmatites such as the White Queen, Oceanside and Elizabeth R. Attractive clear to light blue beryl crystals also occur at pegmatite’s including the Beebe Hole and Pack Rat (Fischer 2002). Topaz is somewhat less common in San Diego County, but does occasionally form excellent, gemmy light blue to clear crystals to 10 cm, most notably at the Little Three mine, which has also produced striking combinations of gemmy spessartine garnet with schorl tourmaline on albite (Fischer 2002).
[caption id="attachment_835" align="aligncenter" width="498"] Blue Topaz: Little Three Pegmatite (photo © Robert Weldon/GIA)[/caption]
Blue Topaz: Little Three Pegmatite (photo © Robert Weldon/GIA)[/caption]
Notable rarer species from San Diego County pegmatites include dark red, lustrous crystals of Stibiotantalite with rynersonite (type locality), both rare tantalum oxides. Fine yellow to brown crystals of danburite, a boron species which is occasionally found in evolved LCT pegmatites, were sometimes found at several pegmatites. Hambergite, a rare boron beryllium siicate, is found in good crystals at several pegmatites as well, only recently eclipsed by spectacular discoveries in Madagascar and Pakistan. While not quite as aesthetic, the rare species boromuscovite was first discovered at the Little Three mine, where it is reasonably abundant. Nice pink to clear crystals of fluorapatite were often associated with quartz, though the most desirable apatites are attached to vibrantly-colored tourmaline crystals, making handsome specimens. Numerous rare phosphate minerals, such as jahnsite and hureaulite also are found in a number of San Diego County pegmatites. Continued mining in San Diego County means that fine mineral specimens will likely be available for many years to come.
10.) Jachymov District, Bohemia, Czech Republic

Jachymov Village in winter (photo © prague-guide.co.uk)
This last, but certainly not least locality was a difficult choice for many reasons, the obvious being that there are dozens of other localities around the world that could be considered ‘top ten’, and I’m many a reader is groaning at this very moment about my failure to include their favorite locality. Another is that there are numerous other ‘world-class’ districts in Europe which are geologically quite similar to Jachymov, such as Freiberg, Germany and Kongsberg, Norway. Nonetheless, I think Jachymov is a worthy addition to the somewhat arbitrary ‘top ten’ list for several reasons. While often forgotten by modern collectors due to scarcity of good specimens on the market and the fact that most mining occurred so long ago, Jachymov is in fact a mineralogical powerhouse, with 429 minerals reported from the district, and 46(!) of these being type localities. Many of these type locality species are not obscure, ugly rarities but in fact globally-important minerals, including uraninite, bornite, and yes, fluorite! This begins to hint at the historical importance of the district in establishing the science of descriptive mineralogy, and the centuries-long mining history there. Additionally, Jachymov is probably the world most important example of the economically-important ‘5 element vein’ type of ore deposit, which will be discussed more in detail later.

Proustite crystal group from Jachymov (4.5 cm across; photo & specimen © Weinrich Minerals)
The Jachymov District (also known as the Joachimsthal District) is situated in the Erzgebirge (literally ‘ore mountains’ in German) mountains, a range of mainly low (<2000 m), rolling, heavily forested mountains near the German border with the northwestern Czech Republic. The history of the district stretches back to the beginning of the 16th century, when the Kingdom of Bohemia ruled the region (Majer & Podlesi 1994). In 1516, rich silver veins were discovered in outcrop at Jachymov, and such a mining rush was created that production at the already well-established mines in nearby Saxony was significantly depressed, and banks and well-to-do merchants all over central Europe scrambled to invest in mining there (Majer & Podlesi 1994). By 1520, over 4000 miners and their families inhabited the ‘free mining town’ of Jachymov, and this rose to over 13,000 by 1525 (Majer & Podlesi 1994). The main purpose of mining at Jachymov in this era was production of silver to provide currency for the Kingdom of Bohemia, and it did so quite well, with over 2.2 million coins already produced by 1528, from 25 open pit and underground mines (Majer & Podlesi 1994). Indeed, even modern currency owes a nod to Jachymov, as the coins minted there were originally called ‘Joachimsthalers’, or ‘thalers’, which was corrupted over time to the word ‘dollars’ (Tyler 1930). Unfortunately, the rich, near-surface silver ores were fairly exhausted by 1550, and Jachymov lay somewhat dormant for a period, though some mining for silver continued, as well as for elements such as nickel and cobalt (then used as a pigment in paints). However, a new boom would come much later, thanks to the presence of a mysterious dense, black colloform mineral the miners called ‘pech blende’ or ‘black ore’, which would turn out to be uraninite (pitchblende). Scientists as early as the great 16th century metallurgist and early mineralogist (though a physician by training) Georgius Agricola noted the ‘bad health effects of inhalation of pech blende dust’, though he had no idea this was caused by exposure to radiation.
[caption id="attachment_838" align="aligncenter" width="580"] Illustration from Georgius Agricola’s seminal 1556 book ‘De Re Metallica’ illustrating mining at Jachymov (photo © fotosearch.com)[/caption]
Illustration from Georgius Agricola’s seminal 1556 book ‘De Re Metallica’ illustrating mining at Jachymov (photo © fotosearch.com)[/caption]
Fast-forward 300 years to the mid-19th century, when it was found that uranium salts, when added to ceramics, could produce beautiful shades of neon green, yellow and red, and the ceramic industry of Bohemia for many years featured brightly-colored enamelware made with such additives (Frame 2015). Later, when Marie Curie isolated the element radium, the rich uranium ores of Jachymov became a main source for this miraculous new element, whose curative powers (when applied to chemotherapy) were recognized early, but whose negative powers (to create the very cancer and other ill health effects it sought to cure) were regrettably not understood until much later. During Victorian times, people all over Europe rejoiced in the supposed curative powers of radium, and items such as radium toothpaste, mouthwash, and ‘5 cent x-rays’ were touted for their health effects (Frame 2015). In 1912, the ‘Marie Curie-Sklodowska Radium Palace’ opened in Jachymov, where visitors could bathe in geothermal springs, which supposedly contained radium, and indulge in various radium health treatments. Unfortunately by the time Marie Curie herself realized the deadly reality of radium, she was already in the advanced stages of terminal thyroid cancer, but her scientific discoveries would and had already changed the world, and would pave the way for the next era of mining at Jachymov. After WWII, when Bohemia and Jachymov descended behind the ‘iron curtain’ of the Soviet Union, Jachymov became a strategic asset, and the larger East German region was the number one supplier of uranium for both energy and nuclear arms to the USSR (Frame 2015). From 1948 to 1962, thousands of laborers, many of them political and other prisoners, labored in the Jachymov mines to extract uranium ore. All of this was highly clandestine, of course, and much is still unknown about Jachymov during this period. By the fall of the iron curtain and the breakup of the Soviet Union in the early 1990’s, mining was mostly gone at Jachymov. Today, the town is somewhat is disrepair, having never economically recovered from the cessation of mining and state planning of every detail of life there during Soviet times, but a modest tourist industry caters to those interested in the fascinating history of the area, as well as several nearby health spa resorts.

‘Pitchblende’ Uraninite, 6 cm across, from Jachymov (photo © Jeff Weissman/mindat.org)
Geologically, Jachymov is part of the great Erzgebirge Mountains metallogenic province, which includes the famous Freiberg district ~100 km away. The main host rocks to the over 200 veins occurring at Jachymov are differentiated granite plutons which have intruded Precambrian metamorphic rocks uplifted during the Hercynian orogeny, mainly schists and amphibolites (Ondrus et al. 2003). The veins are hydrothermal in nature and exploit older faults along which basalt or porphyry dikes related to the granite emplacement occur. The exact timing of mineralization and its relationship to the dikes is not understood, but pitchblende mineralization, associated with carbonates, is thought to be early (close to intrusion of the Hercynian granites around ~300 Ma), and emplacement of silver, cobalt, bismuth and arsenic sulfide ores occurred later, associated with tertiary volcanism (Ondrus et al. 2003). In many cases, earlier, fairly chemically-simple uranium ore veins were overprinted by chemically-complex ores of the ‘5 element’ affinity, containing Ag, As, Bi, Ni, & Co. It is this long-lived and complex mineralizing systems, exploiting the same structural conduits over time, which is probably responsible for the great mineralogical diversity at Jachymov, as we have seen at other world-class localities.
Mineralogically, Jachymov is best known for 3 groups of minerals: silver-containing species, arsenic-containing species, and uranium minerals. While much native silver was mined in the early days, little of it has survived until today, and none of the spectacular wires found at nearby Freiberg were found at Jachymov. However, fine crystals of blood-red proustite to 4 cm, lustrous black acanthite crystals on calcite to several cm, and other secondary silver minerals more than make up for this. Also famous from Jachymov are often large colloform specimens of lustrous black pitchblende uraninite, sometimes in great sizes. While these do not make good specimens to store in your home for obvious reasons, the upside is that the breakdown of primary uraninite at Jachymov resulted in over 80(!) secondary uranium minerals, including colorful examples of zippeite (TL), Metauranopilite (TL), Psuedojohannite (TL), its namesake jachymovite (TL) and more. Additionally, in the ‘5 element veins’, good examples of the classic Ni, Co and Ag arsenides occur, including nickeline, annabergite, rammelsbergite, skutterudite, and safflorite. Finally, a diverse list of secondary arsenates also occur, many forming colorful microcrystals and some in larger crystallized examples. While mining at Jachymov is probably finished, the rich history and mineralogical heritage, from inspiring Agricola’s ‘Der Metallica’ in the 16th century to new mineral discoveries today, will ensure Jachymov is not forgotten.
This article was brought to you by Phillip Persson of PerssonMinerals.com

















































































































 Blue Topaz: Little Three Pegmatite (photo © Robert Weldon/GIA)[/caption]
Blue Topaz: Little Three Pegmatite (photo © Robert Weldon/GIA)[/caption]

 Illustration from Georgius Agricola’s seminal 1556 book ‘De Re Metallica’ illustrating mining at Jachymov (photo © fotosearch.com)[/caption]
Illustration from Georgius Agricola’s seminal 1556 book ‘De Re Metallica’ illustrating mining at Jachymov (photo © fotosearch.com)[/caption]